我看一篇文章说现在一天可做五万测试
没说一天可生产五万台测试机
测试次数和测试机台数 差别挺大的
听听就好了,别认真。美国人还高调说捐赠中国一亿美金呐,到后来,不吱声了。
俺吃过早饭查了一下,新测试机五分钟可以测出阳性结果是真的,但是确实没说得很清楚是一天可生产五万台测试机还是测试机一天可做五万测试(我个人倾向于后者),产生歧义应该是下面这句话:
The company will ramp up its production to make 50,000 units per day beginning next week.
附全文大家看看如下:
The FDA has approved emergency use of a new coronavirus test that delivers positive results in 5 minutes and negative results in 13

- Abbott Laboratories announced Friday that the FDA approved the emergency use of its new Abbott ID NOW COVID-19 test, which can deliver positive test results for the novel coronavirus in five minutes and negative results in 13.
- The test — which is the size of a small toaster — is compact enough that it can be used in any health care setting, including a physician's office or urgent care clinics.
- The company will ramp up its production to make 50,000 units per day beginning next week.
The US Food and Drug Administration granted Emergency Use Authorization (EUA) to a COVID-19 test that delivers positive results in five minutes and negative results in 13 minutes.
Abbott Laboratories claims its
ID NOW COVID-19 test could dramatically change the battle against the novel coronavirus in the US. The test runs on Abbott's ID NOW platform, which is the most common point-of-care test in the US. It is also used to test other viruses including Influenza A&B, Strep A and respiratory syncytial virus (RSV) testing.
The medical device is small and compact enough that it can be used in nearly any healthcare setting, expanding the number of places it can be used to detect the novel virus. The medical device, which is about the size of a toaster, is portable and can be set up anywhere, from a physician's office to an urgent care clinic, the company boasted in a
press release.
"Abbott's ID NOW COVID-19 test will help battle the pandemic in real-time by bringing vital information in minutes to frontline clinicians who are working to stop the spread of the virus," John Frels, vice president of research and development at Abbott Diagnostics, told Business Insider.
After Abbott Laboratories received approval from the FDA for its
ID NOW COVID-19 test on Friday,
the medical device company announced that it would be ramping up its production to make 50,000 units per day as early as next week. According to a spokesperson from Abbott, the tests will be available beginning on April 1.
The FDA also approved Abbott's m2000 RealTime system for coronavirus detection last week. With the two systems combined, the medical device company claims it will produce about 5 million tests for the novel virus in April.
Abbott Laboratories is working with the Trump Administration to deploy the tests to sites where they will have the most impact and will likely target hospital emergency departments, urgent-care clinics, and physicians' offices.
The Abbott ID NOW COVID-19 detects the presence of nucleic acid from SARS-CoV-2 by "identifying a small section of the virus' genome, then amplifying that portion until there's enough for detection."
The molecular testing technology delivers COVID-19 results in minutes. Other coronavirus testing methods require health workers to
take samples from possible coronavirus patients to be delivered to testing labs, taking hours or even days to get results.
However, Abbott n0ted that ID NOW COVID-19 EUA is not FDA cleared or approved, meaning it can only be used by authorized laboratories and patient care settings.
According to Abbott Laboratories, its
ID NOW COVID-19 test is the fastest available molecular point-of-care test. However, other companies producing medical devices with even faster testing capabilities are racing to receive approval from the FDA. Henry Schein Inc. is developing a point-of-care antibody test, however, it cannot definitely diagnose an infection,
Bloomberg reported.
The US government has been criticized for its lagging response to the coronavirus, including its
low testing capabilities after weeks and delays in producing its own coronavirus test. However, the testing capabilities in the US are finally beginning to catch up,
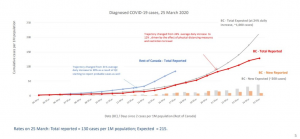
resulting in a surge of confirmed cases across the country going from 32,000 on March 22 to over 100,000 today.
While the US testing capabilities are now in a better place, the healthcare system still faces extreme shortages of critical medical supplies to treat COVID-19 patients, including ventilators and personal protective gear for health workers, including masks.



 先知先觉的好处!冰沟……
先知先觉的好处!冰沟……